Boston Scientific wins CE Mark, FDA nod for smaller 'leadless' ICD
March 17, 2015 by Brad Perriello
Boston Scientific today said it won both CE Mark approval in Europe and a nod at home from the FDA for the latest iteration of its “leadless” implantable cardiac defibrillator, the Emblem S-ICD.
Unlike other ICDs, the S-ICD delivers its regulating shock via subcutaneous leads, rather than with leads threaded through blood vessels to the heart. The Emblem model is 19% slimmer and projected to last 40% longer than the original S-ICD, Marlborough, Mass.-based Boston Scientific said. Read more


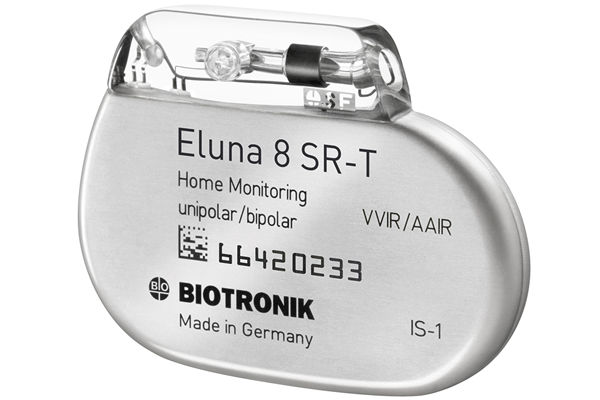
FDA approves Biotronik's Eluna MRI-safe pacemaker
March 20, 2015 by Brad Perriello
Biotronik said today that the FDA approved its ProMRI Eluna MRI-safe pacemaker, making it the only company with single- and dual-chamber MRI-safe pacemakers on the U.S. market.
Lake Oswego, Ore.-based Biotronik said the approval covers the single- and dual-chamber Eluna devices and Setrox leads for full-body MRI. Read more

PAD: Medtronic wins expanded FDA nod for Protégé GPS stent
March 18, 2015 by Brad Perriello
Medtronic today said it won expanded approval from the FDA for the Protégé GPS peripheral stent it acquired along with Covidien earlier this year.
The Protégé GPS self-expanding stent is already approved for use in the superficial femoral and subclavian arteries. The new approval covers its use in treating stenotic lesions in the common and external iliac arteries, Medtronic said. Read more

Australian PE shop acquires Simplify Medical
March 23, 2015 by Brad Perriello
Simplify Medical said it was recently acquired by Australian private equity shop M.H. Carnegie for an undisclosed amount, as it gears up for a U.S. clinical trial of its MRI-safe artifical cervical disc.
“We invested in Simplify Medical because we believe they have a better option for treating patients in a growing market in spine. We are working with the company to accomplish their mission of providing non-metallic, MRI-friendly, anatomically appropriate cervical artificial discs to patients worldwide,” M.H. Carnegie managing director Mark Carnegie said in a statement. Read more
The post BSX wins CE Mark, FDA nod for new S-ICD | Regulatory news for the week of March 23, 2015 appeared first on MassDevice.